Discover the latest guidance on conducting Early-Phase Clinical Trials of Cellular and Gene Therapy Products under an Umbrella Protocol. Learn how to streamline IND submissions and enhance efficiency in your clinical studies.
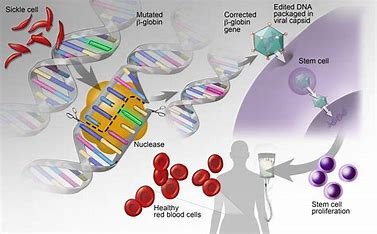
Realizing the promise of cellular and gene therapy.
Addressing Efficiency overheads
Conducting separate clinical trials for different product versions was the norm but it burdened both sponsors and regulatory authorities due to duplicative Investigational New Drug (IND) submissions.
The new guidance is intended to provide a streamlined approach for the assessment of Early-Phase Clinical Evaluation of Cellular and Gene Therapy Products Investigational New Drug Applications (INDs) under an Umbrella Protocol, while allowing sponsors to concurrently evaluate many versions.
Scope and Limitations
The guidance Procedures for Early-Phase Clinical Trials of Cellular and Gene Therapy Products is limited to a single disease.
Basket trials, for example, also do not follow this framework (supports the importance of discussing these issues during pre-submission discussions with OTAT)
Framework implementation for better performance
The most important aspect of the guidance is to classify IND submissions as either “Primary” or “Auxiliary,” thereby simplifying multi-product assessment. Cross-referencing shared materials allows sponsors to streamline duplicative submissions so that the review process becomes more efficient and reduces redundancy of information to limit regulatory burden on both applicant and regulatory agency. .
Strategies for Implementation
Not surprisingly, the guidance stresses the superiority of electronic submissions over paper ones and reminds applicants that they must provide a cover letter (if applicable) as required for any standard submission, emphasizing in-turn whether additional versions or other products should be included. The framework includes guidelines for how to submit amendments and add arms in a specified manner similar to the steps involved with evaluation.
Cell and gene Therapy in the Future
The updated regulations intersect with an era of rapid change in the cellular and gene therapy field, making them a key resource for sponsors and researchers.
Sponsors who follow the accelerated review examination process will simplify administrative responsibilities, shorten novel indications development time and lastly improve on new technology developments for better patient care.
Reach out to BioBoston Consulting or visit our website for more information on how we can help your company.