Discover how Real-World Data (RWD) can transform mRNA immunotherapy development. Learn how to improve efficiency, compliance, and patient outcomes with RWD insights.
In this blog post, we will explore how you can harness the power of RWD to elevate your own efforts in preserving both efficiency and compliance down the path toward transformative healthcare solutions.
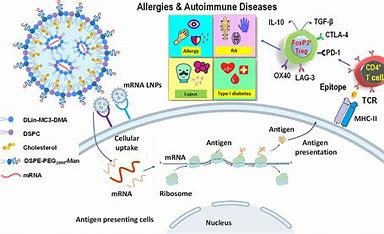
mRNA ImmunoTherapeutics has opened a new era in the life science industry, offering an unprecedented opportunity to treat diseases at their genetic origins.
But there are hurdles on the road to success, and regulations around it change all the time. To successfully traverse this complex landscape, industry stakeholders must first be innovative and secondly adhere to strict guidelines in terms of quality as well compliance.
Real-World Data (RWD) Explained
Real-World Data — observational data which are drawn from real-world sources as opposed to the rigid and controlled environment of a clinical trial. Utilizing RWD offers a more holistic picture of how well or poorly a treatment performs in real-world conditions than any other means as it provides an indication on its efficacy, safeness, and long-term effects.
For a Quality and Regulatory Consulting firm, the extent of this data is recognized to provide real-world evidence that acts as a bridge between clinical trials finish line with day in/out application.
Speeding Up Development with RWD Insight
Time is of the essence in mRNA immunotherapy. Using Real-World Data enables organizations to accelerate the process of development by identifying patterns, trends, and any obstacles at an early stage.
By being proactive as opposed to reactive, decision-making is streamlined and adaptability which is key in the rapidly evolving field of immunotherapy development is improved.
Compliance With Regulations
There has been an increasing acknowledgment by regulatory agencies of the value that Real-World Data (RWD) hold to determine safety and effectiveness in novel approvals.
Here at BioBoston, a consulting firm specialized in helping life science organizations navigate the complexity of regulatory compliance; we help ensure that when RWD is used, it is done both effectively and responsibly based on rapidly changing norms.
Why It Matters: Reducing Risks, Improving Patient Outcomes
Our consultancy works with you to minimise risks in your mRNA immunotherapy development strategy by including Real-World Data.
This not only protects your work but also improves patient care by offering therapies that are innovative and suits real-world needs and requirements.
As a Quality and Regulatory Consulting firm leaders in the mRNA immunotherapy space, we know where this is all headed: It is at innovation through compliance.
By using Real-World Data, your organization can traverse the maze of development with confidence leading to revolutionary healthcare advancements.
Conclusion: Partnering for Success in Healthcare
Join us on this journey and partner with us to help define a future where mRNA immunotherapies are set alight as the new standard of care.
Contact BioBoston Consulting today or go to our web site for more details on how we can help your organization.