Navigating Health Canada’s medical device classification system is crucial for ensuring safety and effectiveness. Explore this important regulatory framework with our life science consulting experts.
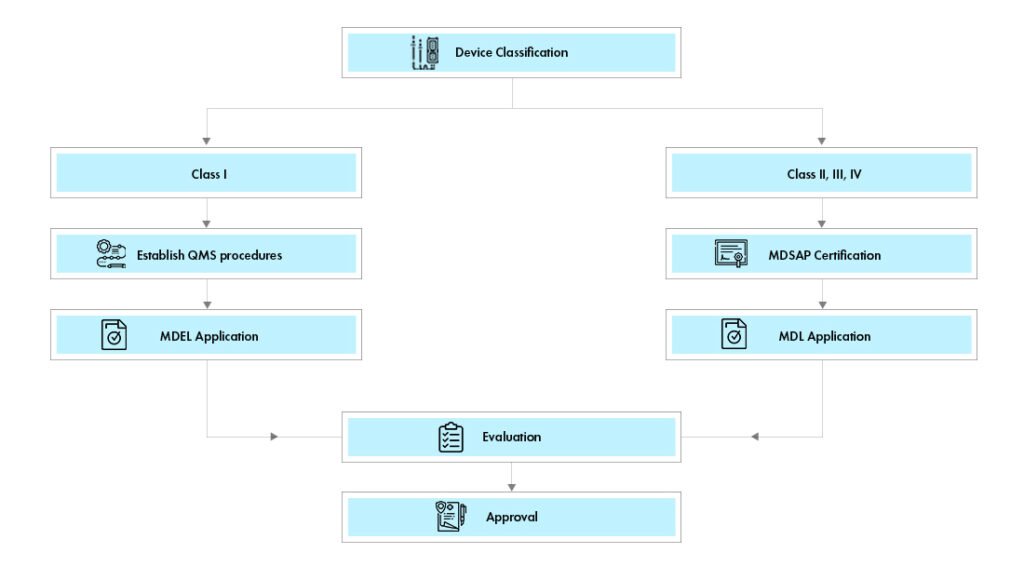
Health Canada administers the regulation of medical devices in Canada, including establishing a classification system for those devices based on their inherent risks. For instance, it ensures that medical devices are properly controlled to avoid harming the health and safety of Canadians. In this report, we take a detailed look into specific
The Medical Device Classes
Health Canada classifies all medical devices into four classes, with Class I having the lowest risk to Class IV having the highest. Certain factors determine the classification of a medical device, such as the intended use of the device, the potential harm associated with the device, as well as how invasive the device is.
Current Good Manufacturing Practice:
According to the cGMP regulations, pharmaceutical manufacturing facilities are to be compliant with general principles established under Title 21 and Part 211. Section 211.44 details that adequate lighting shall be provided throughout the facilities. Therefore, this provision is really underlined for the purpose of placing significant emphasis on how illumination ser
Class I Devices
Class I products are least risky and least under legal control. This type of medical equipment does not involve threatening damage to the human body. Examples of Class I devices include bandages, syringes, stethoscopes, among many others. ves as one of the integral factors for safety and quality in the manufacturing of pharmaceutical products.
Class II Devices
Class II devices are moderate risk devices. These are more regulated than class I devices. These devices also pose a moderate risk and, therefore, can harm the user with moderate to high potential invasion. Some examples of class II devices are ultrasound machines, X-ray equipment, and powered wheelchairs.
Class III Devices
Class III devices are high-risk and subject to extensive regulation. These devices are invasive and pose a serious risk to the user. This class of devices includes pacemakers, implantable defibrillators, and artificial heart valves.
Class IV Devices
Class IV devices have the highest risk and are the more strictly regulated. These include implantable devices that can cause fatal harm to a person if they malfunction. Such devices include neurostimulators and spinal cord stimulators.
Requirements of Classifications
For any medical device destined for Canada, manufacturers must submit an application to Health Canada, providing comprehensive information about the device, its intended use, and any hazards it may pose to users. This information, along with other relevant considerations deemed necessary by Health Canada, will then decide the classification of the device.
Apart from the classification of the device, manufacturers should ensure that their device complies with all relevant regulatory requirements for its class. This includes safety, performance, and labeling requirements.
Conclusion
The classification system provided by Health Canada for medical devices is a crucial tool that guarantees the safety and effectiveness of medical devices in Canada. Manufacturers need to understand the specific requirements for the classification of their devices and ensure they satisfy all the regulatory needs of their device’s class. At this point, it is prudent to ensure that the device produced meets minimum health, safety, and efficacy standards for safe and effective use by Canadians.
Contact BioBoston Consulting today or check our website to get in touch with us and learn more about how we can add value to your organization.