Learn the essential steps to create a comprehensive Pharma Validation Master Plan with BioBoston Consulting Services. Ensure regulatory compliance and quality control in pharmaceutical manufacturing.
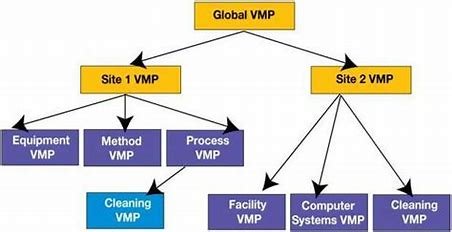
In the pharmaceutical industry, ensuring product quality, safety, and compliance with regulatory standards is essential. One of the most critical documents in this process is the Pharma Validation Master Plan (VMP). A well-structured VMP outlines the strategy for validating processes, equipment, and systems, ensuring that products meet the required standards throughout their lifecycle.
However, creating a comprehensive and effective VMP can be a daunting task for many pharmaceutical companies. This is where BioBoston Consulting can help. Our team of experts offers tailored pharma validation services to guide your company through each phase of developing and implementing a successful Validation Master Plan.
What is a Pharma Validation Master Plan (VMP)?
A Pharma Validation Master Plan (VMP) is a critical document that outlines the approach for validating processes, systems, equipment, and software used in pharmaceutical manufacturing. It serves as a roadmap for achieving compliance with Good Manufacturing Practices (GMP) and other regulatory requirements. The VMP provides the foundation for ensuring product quality, safety, and efficacy, which are essential to the success of any pharmaceutical product.
Why is a Pharma Validation Master Plan Important?
A well-designed VMP ensures:
- Regulatory Compliance: It helps meet FDA regulations, EU GMP standards, and other local or international guidelines.
- Risk Mitigation: Identifies potential risks early in the manufacturing process, ensuring that these risks are effectively managed.
- Product Quality: Ensures that products are consistently manufactured to meet quality standards and specifications.
- Operational Efficiency: Streamlines processes, reducing unnecessary delays and minimizing cost overruns in production.
Essential Steps in Creating a Pharma Validation Master Plan
The creation of a Pharma Validation Master Plan involves several critical steps listed below, which are vital to the success of the validation process.
1. Define the Scope and Objectives
The first step in developing a VMP is to clearly define the scope and objectives of the validation process. This includes identifying which systems, processes, and equipment will be validated. It is essential to understand the purpose of each validation to ensure a comprehensive and well-targeted plan.
BioBoston Consulting helps companies define the scope of their VMP based on industry-specific needs, regulatory requirements, and best practices.
2. Develop a Validation Strategy
Once the scope is determined, the next step is to develop a validation strategy. This includes choosing the types of validation needed, such as process validation, equipment validation, software validation, and cleaning validation. It also involves identifying the acceptance criteria and methods to evaluate whether each system or process meets the required standards.
BioBoston assists pharma companies in designing and implementing effective validation strategies that align with regulatory guidelines and company goals.
3. Conduct Risk Assessment
A comprehensive risk assessment is essential to identify potential risks that may impact product quality and compliance. By evaluating the likelihood and impact of these risks, BioBoston ensures that appropriate mitigation strategies are implemented in the validation plan.
BioBoston Consulting’s experts use risk-based approaches to assess and mitigate risks, focusing on areas that are most critical to product safety and regulatory compliance.
4. Create a Detailed Validation Protocol
The next step is to create a validation protocol, which outlines the specific activities, tests, and documentation required to validate each system or process. This protocol should include acceptance criteria, testing procedures, timelines, and responsible personnel.
BioBoston’s team works closely with pharmaceutical companies to develop clear, thorough validation protocols that ensure the process is repeatable and auditable, while also meeting regulatory standards.
5. Execute Validation Activities
With the validation protocol in place, the next step is to execute the validation activities. This includes testing equipment, processes, and systems to verify they meet the requirements set out in the VMP. The execution phase is critical for identifying any issues that may need to be addressed before full-scale production can begin.
BioBoston Consulting’s professionals oversee and guide the validation execution, ensuring each activity is performed according to the agreed protocols and meets both internal and regulatory requirements.
6. Document and Review Results
Documentation is a vital part of the pharma validation process. Every step, test, and result must be documented clearly, traceable, and compliant with Good Manufacturing Practices (GMP). After all activities are completed, a thorough review of results is necessary to confirm that everything meets the acceptance criteria.
BioBoston ensures that all validation activities are thoroughly documented, and the results are reviewed to guarantee that the products meet the highest standards of quality and compliance.
7. Prepare for Regulatory Inspections
Once the VMP is executed, the final step is to prepare for regulatory inspections. Regulatory bodies such as the FDA, EMA, and other national authorities may review your validation documents to ensure compliance with their standards. BioBoston’s consulting services help you prepare for inspections by ensuring that your VMP and related documentation are in order and ready for review.
With our expertise, BioBoston ensures that your VMP passes the scrutiny of regulatory inspections, avoiding delays and maintaining compliance with GMP.
Why Choose BioBoston Consulting for Pharma Validation Master Plans?
- Expert Guidance: BioBoston Consulting brings in-depth knowledge of pharma validation and regulatory compliance to help your company navigate complex processes.
- Tailored Services: We provide personalized consulting services based on your company’s specific needs, ensuring that your Pharma Validation Master Plan aligns with both regulatory requirements and business objectives.
- Proven Track Record: With years of experience in the pharmaceutical industry, BioBoston has successfully helped numerous clients develop and implement robust validation master plans.
- Comprehensive Support: From risk management and process validation to audit readiness, BioBoston offers comprehensive support throughout the entire validation lifecycle.
Contact BioBoston Consulting Services Today
If your pharmaceutical company is ready to develop a robust Pharma Validation Master Plan that ensures regulatory compliance and product quality, BioBoston Consulting Services is here to help. Our experts are equipped with the knowledge and experience to guide you through every step of the validation process.
Contact us today to schedule a consultation and learn more about how BioBoston can help your company stay compliant with the latest pharma regulations and ensure product success.