Learn everything you need to know about clinical trial protocols, including key components, challenges, and trends in protocol design. Contact BioBoston Consulting for expert guidance in clinical trial planning.
The Essential Guide to Clinical Trial Protocols
Clinical trial protocols are the foundation of every successful clinical trial. They outline a systematic plan for executing the trial, specifying each phase — from patient recruitment and dosage to monitoring, safety assessments, and data collection. If you are a pharmaceutical company, CRO or clinical Investigator, it is important for you to know the essentials of clinical trial protocols for promoting the trial, commercializing it and to ensure compliance and safety.
We will explore clinical trial protocols, look at the key basic and critical elements that make up a protocol, understand the common challenges in protocol development, and discuss the new trends and designs in clinical trial protocols. If you want expert advice on creating or optimizing your clinical trial protocols, BioBoston Consulting can help streamline this process for you.
What Is a Protocol for a Clinical Trial?
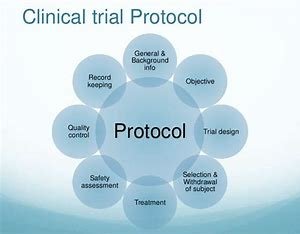
The clinical trial protocol is an extensive, detailed document that formalizes the trial-objectives, methodology, design & operational details. It serves as a “blueprint” for the study, ensuring that all participants, investigators and sponsors follow the same plan. The protocol outlines every detail of the trial, including:
Study Objectives: The goal of the trial is to understand if a drug or treatment works.
Patient Inclusion/Exclusion Criteria: Patient inclusion/exclusion criteria.
Study Design: The framework of the study, encompassing randomization and blinding, and control methods.
For the investigational product: dosage and administration.
Main Outcome Measures: The key endpoints used for the success of the treatment or intervention.
Statistical Methods: How the data will be analyzed to prove the success of the trial.
Not only does a properly written protocol guarantee satisfaction of regulatory agencies, but it acts as a reference point for investigator, sponsor, and research teams during the trial process.
Ingredients of Clinical Trial Protocols
Study Design
One critical aspect of a clinical trial protocol is the study design. It will describe how the trial will be conducted so that it will answer the specific research question clearly and efficiently. This includes details on:
Randomisation: If participants will be randomised to treatment groups.
Blinding: Whether the study is double-blinded (neither the patient nor the investigator knows which treatment is being given) or open-label
Control Groups: If there is a placebo or standard treatment group for comparison.
Patient Selection Criteria
Defining clear inclusion and exclusion criteria is crucial so that participants fulfil the required health conditions and will further the validity of the trial outcomes. These fields guide in reducing the number approaches to bias.
Dosage and Treatment Regimen
The protocol will detail how the investigational treatment will be given, the dose, the number of times it will be given, and for how long. It is a vital step in assessing the drug or treatment’s safety and effectiveness.
Endpoints and Outcome Measures
Endpoints are the measures the trial will use to judge whether the treatment works. These may be clinical outcomes such as disease progression, survival, or disease biomarkers. Well-defined primary and secondary endpoints make sure the goals of the trial are reached and that there are meaningful results.
Statistical Methods
The protocol should specify how the data from the trial will be analyzed. There are statistical methods to help ensure that the conclusions drawn from the trial are valid and reliable. In this section, sample size calculations, randomization procedures, and statistical tests used to compare outcomes are described.
Typical Issues Faced in the Process of Developing Clinical Trial Protocol
Regulatory Compliance
One of the biggest challenges is to ensure the clinical trial protocol complies with regulations from health authorities such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA) and other local agencies. Guidelines are also not standardised and will differ based on the region, often changing over time so staying updated with current guidelines will be key.
Solution: Work with regulatory experts, like BioBoston Consulting, to develop your protocol in such a way that meets all of the regulatory requirements and avoids delays or complications.
Patient recruitment and retention
Finding the right patients and maintaining their enrollment throughout the study can be a significant hurdle. Inclusion and exclusion criteria should be balanced: overly stringent criteria can result in under-enrollment while loose criteria can compromise the integrity of a trial.
For example, effective patient-centric recruitment strategies provide solutions to some commonly faced issues that contribute to poor recruitment and retention rates.
Budget and Time Constraints
Clinical trials are expensive, and the protocols must be designed for efficiency. This can lead to cost overruns in those trials or an extended trial timeline if the interim analysis is flawed — they underestimate how much they will need, or they fail to take delays into account.
Solution: Allow clinical trials to be planned flexibly and adapt if something goes wrong.
Data Management
Clinical trials involve data collection, storage and analysis. Bad data management systems can result in inconsistencies, errors and delays. Clinical trials can produce a lot of data, so it is important to have a strategy for data management and analysis in place.
Solution: Implement electronic data capture (EDC) systems and online data management solutions to improve data accuracy and expedite real-time information sharing.
Evolution of Clinical Trial Protocol Design
Patient-Centric Trial Design
The evolution of patient-centric trials requires the design of less rigid protocols that are more attractive to the patients. This includes decentralized trials (DCTs), which enable remote participation for patients, and adaptive trial designs that modify the protocol based on interim results.
Trend: More flexible protocols can allow for personalized treatments and decrease patient burden, improving recruitment and retention.
Employment of Technology and Automation
AI, ML, and real-time data analytics are revolutionizing clinical trial design and results. These technologies can help optimize protocols, enhance recruitment as well as improve monitoring and compliance.
Trend – Trial Design Optimization: AI and Data-Driven Approaches
Incorporating Real-Time Data
We will also be able to accelerate catchup and diversity with wearable devices and remote monitoring tools that can build real-time data to significantly enhance clinical trials. This dynamic nature of trial protocol allows studies to be adapted according to the real time ongoing patient data, creating potentially more efficient studies.
Trend: Real-time data collection enhances patient safety and the efficiency of clinical trials through continuous insights.
Conclusion
The success of any clinical research study hinges on clinical trial protocols. This knowledge on the key points of protocol design, as well as on the trial field happenings allows you to make the most out of existing resources, while at the same time ensuring patient safety, regulatory compliance and results that can be trusted. On the flip side, if you want to develop a robust clinical trial protocol, it is not without its challenges, ranging from regulatory requirements, patient recruitment, to data management. And that is the role BioBoston Consulting stepped into.
At BioBoston Consulting, we focus on the process of prototyping, coding, and piloting clinical trial protocols. However, there is a lot to navigate when it comes to planning a clinical trial, and our experienced and skilled team will take you through the process to make sure your study goes smoothly and efficiently.
We can help with clinical trial protocol design or optimization. We will ensure your trial not only meets all regulatory requirements but is also delivered on time and produces reliable, actionable results. Contact today and move ahead toward effective clinical research!