Discover the key challenges and strategies for transitioning from Phase 2 to Phase 3 in drug development. Learn about clinical trials, patient recruitment, regulatory compliance.
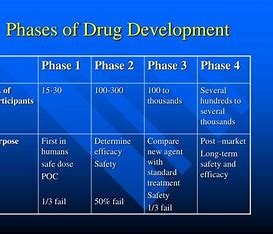
The shift between phase 2 and phase 3 drug development is one of the most critical steps in the process of getting a drug to market. The aim of phase 2 is to provide demonstration of proof of concept of the Phase II molecule and establish the appropriate dose and dosing regimen. Phase 3 (however) is focused on efficacy and safety in larger patient populations. Moving from phase 2 to phase 3 typically presents a challenge because there is a lot of planning and preparation needed for the drug to be ready for the greater patients and regulatory scrutiny associated with phase 3. This article will highlight some of the issues pharma companies will face as they move from phase 2 to phase 3, and how to avoid some of the pitfalls.
Clinical Trial Design and Execution
A central challenge of moving from phase 2 to phase 3 is to design and execute a clinical trial that is optimally powered to confirm efficacy and safety in a larger patient population. One of the key challenges is to make sure that the study protocol is set up in a way to answer relevant scientific questions and confirm that the trial adheres to the protocol. If not set up properly, the trials could lead to non-determinate data, which may prolong the approval process.
As regulatory requirements become stricter, this can be difficult for a pharmaceutical company to navigate, especially with Phase 3 clinical trials being under the heaviest scrutiny. Regulatory agencies command a wealth of data before deeming safety and efficacy, a process that can take years and dollars to collect. Both specific countries and regions have different regulatory requirements that can make it challenging for companies to navigate through complexities of regulations.
Patient Recruitment
Recruiting Patients for Phase 3 clinical trials can be difficult, especially if the disease under investigation is uncommon. This means pharmaceutical companies need to have a solid recruitment strategy in place to enroll enough patients to fill the study requirements. It may include collaborations with patient advocacy organizations, segmented campaigns, and outreach with providers to identify qualifying patients.
Manufacturing And Supply Chain:
During transition of phase 2 to phase 3 itself, there can be few manufacturing and supply chain issues as drug product must be made in large volume. The pharmaceutical company must be able to manufacture and supply the drug as needed for the clinical trial. Aspects of this must be carefully planned and executed such that not only the drug itself is produced to the requisite quality, but the associated supply chain is resilient and dependable.
Phase 3 clinical trials
It can be resource-intensive, with costs often in the millions of dollars. Pharma companies need to be able to afford and able to conduct the clinical trial. Budget needs to be planned and managed well for funds to be utilized properly.
The pharmaceutical industry must prepare diligently with a full transition plan that covers all these challenges. It should cover key elements of clinical trial design and execution, regulatory strategy, patient recruitment, manufacturing and supply chain, and finance.
How can experienced consultants and service providers support pharmaceutical companies manage the transition from phase 2 to phase 3?
These experts could help on clinical trial design and execution, regulatory issues, patient recruitment and manufacturing and supply chain issues.
Moving from phase 2 to phase 3 of drug development
It can be a daunting prospect for pharmaceutical companies. From clinical trial design and execution to regulatory compliance, patient recruitment, manufacturing and supply chain, and financial management, the pharmaceutical company must make sure their drug is ready for the even larger patient populations and clinical scrutiny associated with phase 3.
Learn how we can support you: Contact BioBoston Consulting here or visit our website.