Learn about the Commissioning, Qualification, and Validation (C&Q) process for regulated industries like pharmaceuticals, biopharma, and biotechnology.
In regulated industries, understanding the distinctions between Commissioning, Qualification, and Validation (C&Q) is essential for maintaining high standards of safety, quality, and compliance. By exploring the unique roles of each, companies can ensure that their processes, systems, and equipment are always ready for operation, compliant with regulations, and capable of delivering consistent results.
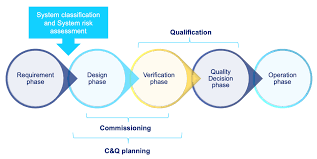
What is Commissioning, Qualification, and Validation?
The processes of Commissioning, Qualification, and Validation (C&Q) are integral to ensuring that facilities, systems, and equipment are fit for their intended purposes. These terms, while occasionally varying in definition, all describe stages of verification and testing that are crucial to regulated industries like pharmaceuticals, biopharma, and biotechnology.
Validation offers documented evidence that processes will consistently produce products that meet the defined specifications. Both Commissioning and Qualification can be seen as subsets of validation, but they focus on different parts of the lifecycle. Here is a closer look at the key differences between them:
Commissioning: Engineering the Foundation for Safe Operations
Commissioning involves a structured, documented, and managed approach to the startup and turnover of facilities, systems, utilities, and equipment. The goal is to ensure that everything works as expected and meets predefined design and safety standards. Typically, Commissioning is seen as more of an engineering exercise, where construction and engineering teams verify the integrity and functionality of the systems as they are being installed.
During the Commissioning phase, engineers and subcontractors focus on:
- Inspecting systems to ensure they meet engineering specifications.
- Verifying that all equipment is correctly installed.
- Ensuring that utilities (water, air, power) function appropriately.
- Conducting risk assessments for the system’s safe operation.
Qualification: Ensuring Systems Meet Functional Specifications
Following Commissioning, Qualification comes into play to confirm that the systems, equipment, and processes are operating within predefined specifications and performing as intended. Qualification includes rigorous testing and documentation of the system’s reliability, performance, and compliance with regulatory standards.
Unlike Commissioning, which focuses on the physical setup, Qualification emphasizes the system’s actual operational performance under real-world conditions. Qualification is often divided into three stages:
- Installation Qualification (IQ): Verifying that equipment is installed correctly and in compliance with design specifications.
- Operational Qualification (OQ): Confirming that equipment operates as intended across all specified operating ranges.
- Performance Qualification (PQ): Ensuring that the system functions effectively under routine production conditions.
The quality unit usually oversees the Qualification process, ensuring all necessary tests, procedures, and documentation are complete and compliant with industry regulations.
Validation: Ensuring Consistent Quality and Compliance
The final stage, Validation, is about generating robust evidence that a process will consistently produce high-quality products that meet established quality attributes. It involves rigorous documentation of the process, ensuring that it can reliably deliver the intended product output under various conditions.
Validation extends beyond the initial setup of systems and equipment and continues to ensure ongoing consistency and compliance with regulatory standards. This includes:
- Validation protocols that are designed and executed to document each critical aspect of the manufacturing process.
- Ongoing monitoring and control after the equipment and systems are in use, as part of Continued Process Validation (CPV).
- Control strategy maintenance to safeguard product quality throughout the lifecycle.
Commissioning vs. Qualification: Key Differences
To break it down visually, here is a comparison between Commissioning and Qualification:
| Aspect | Commissioning | Qualification |
| Focus | Equipment and system installation and functionality | Operational readiness and compliance with requirements |
| Acceptance Criteria | Equipment installation and configuration | Product and performance requirements |
| Testing | Initial adjustments, inspections, and tests | IQ, OQ, and PQ (Installation, Operational, and Performance Qualification) |
| Oversight | Engineering and construction teams | Quality units, regulatory compliance teams |
The Continuous Role of Validation in CQV
After Commissioning and Qualification are completed, Validation ensures that the systems and processes continue to meet their intended purpose. The validation process does not end once initial tests and trials are completed. Instead, a continuous assessment of performance is required to ensure ongoing compliance and the consistency of product output.
Continued Process Validation (CPV) ensures that systems remain within defined specifications throughout their lifecycle, mitigating the risk of deviations that could affect product quality or regulatory compliance. This is a critical part of maintaining long-term compliance and operational excellence.
Partnering for Success in CQV: How BioBoston Consulting Can Help
Navigating the complexities of Commissioning, Qualification, and Validation requires expertise and thorough understanding of both engineering principles and regulatory requirements. At BioBoston Consulting, we specialize in helping businesses in regulated industries streamline their CQV processes. We provide comprehensive services that ensure your systems and processes not only meet stringent industry standards but are continuously optimized for safety and efficacy.
By partnering with BioBoston Consulting, you gain access to:
- Expert advice on Commissioning, Qualification, and Validation for all types of regulated systems.
- Tailored strategies to ensure compliance with FDA, European Commission (EC), and global regulatory bodies.
- Comprehensive CQV protocols and documentation that streamline your path to operational excellence.
- Ongoing support for Continued Process Validation (CPV) to maintain regulatory compliance and product quality.
We are committed to guiding you through every stage of the CQV process, ensuring that your systems and processes are not only compliant but perform at their best.
Get in touch with our team at BioBoston Consulting today to discuss how we can help you optimize your Commissioning, Qualification, and Validation processes for compliance, efficiency, and product quality.