Discover how pharmaceutical companies can leverage ICH Q14 for enhanced analytical procedure development, validation, lifecycle management, and global regulatory compliance. Learn how BioBoston Consulting can support your implementation.
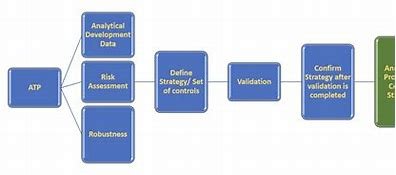
The ICH Q14 guideline offers pharmaceutical companies a structured and comprehensive approach to analytical procedure development, validation, and lifecycle management. By adopting these principles, companies can enhance their processes, minimize risks, and ensure product quality and compliance. Here’s how pharmaceutical companies can leverage ICH Q14 to stay ahead in the industry:
1. Streamlined Analytical Method Development
By aligning with ICH Q14, pharmaceutical companies can design tailored analytical methods specific to their products and processes. This results in robust, efficient, and reliable methods, reducing the risk of errors and ensuring consistency across all stages of production.
2. Robust and Reliable Method Validation
ICH Q14 helps companies establish clear acceptance criteria for method performance, ensuring methods are validated based on scientific principles. This results in thorough validation, reducing the likelihood of method failures or inaccuracies that could harm product quality.
3. Proactive Risk-Based Approach
The risk-based approach promoted by ICH Q14 enables companies to identify and address potential risks early in the method development process. By managing these risks proactively, companies can develop more reliable methods and avoid costly regulatory hurdles.
4. Built-in Quality with Quality by Design (QbD)
Implementing Quality by Design (QbD) principles into analytical procedures ensures that methods are optimized for performance and regulatory compliance from the outset. This approach reduces the need for post-approval changes by ensuring that methods are already designed with quality in mind.
5. Continuous Lifecycle Management
Managing the lifecycle of analytical procedures as recommended by Q14 allows companies to optimize methods throughout a product’s lifecycle. This ongoing improvement leads to cost savings and enhanced product quality as processes are refined over time.
6. Flexibility in Managing Post-Approval Changes
The Q14 guideline facilitates post-approval changes to analytical procedures by employing a risk-based approach. This flexibility helps companies make efficient adjustments while maintaining product quality and regulatory compliance, reducing the need for extensive revalidation efforts.
7. Alignment with Global Regulatory Standards
Adhering to ICH Q14 ensures that analytical methods comply with international regulatory expectations, which helps streamline the regulatory approval process and opens the door for global market access. This alignment gives companies a competitive advantage on the international stage.
8. Maximizing Resource Efficiency
By using tailored analytical methods, pharmaceutical companies can reduce resource-intensive activities such as retesting, method optimization, and unnecessary validations. This optimization leads to better resource utilization, saving both time and money.
9. Ensuring Data Integrity
Following ICH Q14 ensures that the data generated from analytical procedures is accurate, reliable, and compliant with regulatory standards, reducing the risks of data integrity issues that could negatively impact product quality and regulatory standing.
10. Fostering a Culture of Continuous Improvement
The Q14 guidelines encourage a continuous improvement mindset, where companies regularly review and refine their analytical procedures. This practice leads to higher efficiency, better product quality, and a competitive edge in a constantly evolving market.
11. Facilitating Seamless Technology Transfer
The Q14 framework simplifies the transfer of analytical methods across laboratories or to contract organizations, enabling a smoother technology transfer process. This minimizes delays in product development and accelerates market commercialization.
12. Staying Ahead with Scientific Advancements
As analytical technologies evolve, Q14 provides a framework for adopting new technologies and methodologies, helping companies stay at the forefront of analytical capabilities and ensure that their procedures remain in line with the latest scientific developments.

Achieve Excellence with ICH Q14: How BioBoston Consulting Can Help
Integrating ICH Q14 into your operations offers numerous benefits, from improved method quality and reduced risks to better regulatory compliance and operational efficiency. By embracing these guidelines, pharmaceutical companies can ensure consistent product quality, meet regulatory requirements, and stay ahead in a competitive market.
At BioBoston Consulting, we specialize in helping pharmaceutical companies navigate the complexities of regulatory compliance, including the adoption and implementation of ICH Q14. Our team of experts can guide you through every stage of analytical procedure development, validation, and lifecycle management, ensuring that your methods meet the highest standards.
Our Expertise Includes:
- Developing and validating robust analytical methods that align with ICH Q14 principles.
- Implementing risk management strategies to identify, mitigate, and monitor potential risks in your processes.
- Driving continuous improvement initiatives to keep your methods up to date with evolving industry standards.
If you are ready to streamline your analytical procedures and ensure regulatory compliance with the ICH Q14 guidelines, BioBoston Consulting is here to help. Contact us today to learn more about how we can assist in optimizing your analytical methods and boosting your product quality for global market success!