Learn about the ICH Q14 guidelines for developing and validating analytical procedures in pharmaceutical manufacturing. Discover how BioBoston Consulting can assist with ICH Q14 implementation and ensure compliance.
The ICH Q14 guideline is set to significantly enhance the way pharmaceutical companies approach analytical procedure development. It provides a comprehensive framework for developing and validating analytical methods to ensure the accuracy, consistency, and regulatory compliance of drug substances and products. By improving data quality and streamlining processes, ICH Q14 aims to drive greater efficiency and innovation in drug development and manufacturing.
What is the ICH Q14 Guideline?
The ICH Q14 guideline outlines the science and risk-based approaches for developing analytical procedures used in testing the quality of drug substances and products. The guideline applies to testing during clinical development, release, and stability testing of both commercial drug substances and final drug products. It complements earlier ICH guidelines like ICH Q2 (Validation of Analytical Procedures), and integrates principles from ICH Q8 (Pharmaceutical Development), ICH Q9 (Quality Risk Management), and ICH Q12 (Lifecycle Management).
The goal of ICH Q14 is to develop analytical procedures that are fit for their intended purpose—whether that is measuring a specific attribute of a substance or assessing product quality with the necessary specificity, accuracy, and precision.
Two Approaches to Analytical Procedure Development
ICH Q14 outlines two main approaches to developing analytical procedures:
1. The Minimal Approach
The minimal approach emphasizes establishing a simple, robust, and well-defined analytical procedure that is fit for its intended purpose without the need for excessive optimization.
Key elements include:
- Robustness Testing: Ensuring the procedure is stable and reliable.
- Method Suitability: Evaluating the procedure for specificity, linearity, accuracy, precision, and detection limits.
- Reference Standards: Utilizing proper standards to ensure the procedure’s accuracy and reliability.
- Stress Testing: Conducting tests to assess how well the procedure detects degradation products.
- Minimum Testing: Focus on the least amount of testing needed to confirm the method’s suitability and reliability.
2. The Enhanced Approach
The enhanced approach involves more extensive method development and optimization, leveraging modern statistical tools and quality management principles to better understand the method’s critical parameters.
Key elements include:
- Design of Experiments (DoE): Systematic application of statistical tools to optimize method parameters.
- Method Optimization: Adjusting various factors such as sample matrix and robustness to improve method performance.
- Quality by Design (QbD): Creating a comprehensive understanding of critical attributes to ensure consistent method performance over time.
- Risk Assessment: Identifying and managing risks associated with the method’s performance.
- Lifecycle Management: Considering the entire life cycle of the analytical method, from development to post-approval changes.
Key Elements of the ICH Q14 Guidelines
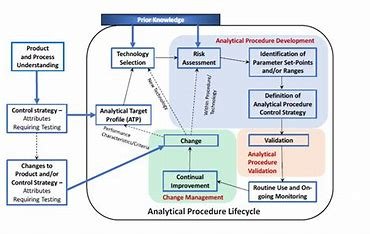
1. Analytical Procedure Lifecycle
The Analytical Procedure Lifecycle in ICH Q14 is divided into three stages:
- Procedure Design: The initial phase of developing the analytical method.
- Procedure Performance Qualification (PPQ): The stage where the procedure is validated and proven to be reliable and robust.
- Continued Procedure Performance Verification (CPV): Ensuring the procedure remains effective and compliant throughout its lifecycle.
2. Analytical Target Profile (ATP)
The Analytical Target Profile (ATP) is a crucial foundation for developing an analytical procedure. It defines the procedure’s intended purpose, the attributes to be measured, and its performance characteristics. The ATP guides the selection of technologies, methods, and validation strategies.
3. Knowledge and Risk Management
ICH Q14 encourages the use of knowledge management—both internal (company experience) and external (scientific publications, established principles)—to inform the development of analytical procedures. By applying quality risk management, the guideline helps minimize risks associated with method performance and ensures more reliable results.
4. Evaluation of Robustness and Parameter Ranges
Robustness evaluation assesses the method’s ability to perform as expected under normal operational conditions. By deliberately varying parameters, the procedure’s robustness is tested. Knowledge from previous risk assessments can inform the parameters to investigate, and parameter ranges can be determined through univariate or multivariate experiments.
5. Analytical Procedure Control Strategy
A Control Strategy ensures that the analytical procedure is performed consistently during routine use. It includes parameters that need to be controlled and system suitability tests (SST). The Analytical Procedure Control Strategy also ensures that sample suitability assessments are carried out when needed.
In line with ICH Q12, applicants may propose established conditions (ECs) for the procedure, which are then reviewed and approved by regulatory authorities.
6. Lifecycle Management and Post-Approval Changes
Changes to analytical procedures during the product’s lifecycle must be carefully managed to ensure continued compliance. Risk assessment plays a critical role in determining the nature of changes and the appropriate reporting category for these changes. Bridging strategies can be implemented to demonstrate the ongoing suitability of the method post-change.
Conclusion: ICH Q14 and Its Impact on the Pharmaceutical Industry
ICH Q14 promises to be a game-changer for the pharmaceutical industry by offering a structured approach to analytical procedure development. Through its emphasis on risk-based approaches, robust testing, and continuous performance verification, ICH Q14 ensures that analytical procedures remain reliable and compliant, driving better product quality and patient safety.
For pharmaceutical companies, adopting ICH Q14 guidelines will enhance the efficiency of drug development processes, ensure regulatory compliance, and foster innovation across the industry.
How BioBoston Consulting Can Assist with ICH Q14 Implementation
At BioBoston Consulting, we specialize in helping pharmaceutical companies implement and adhere to ICH Q14 guidelines. Our expert team can guide you through the intricacies of analytical procedure development and ensure compliance with international standards.
Our services include:
- ICH Q14 Guidance: We provide expert advice and support in aligning your analytical procedures with ICH Q14 requirements.
- Risk Management Strategies: Our team can help identify and mitigate risks in your analytical methods, ensuring consistent results.
- Method Optimization: We assist in optimizing your analytical procedures for maximum efficiency and accuracy.
- Lifecycle Management: We ensure that your procedures are continuously monitored and improved to maintain regulatory compliance.
Contact BioBoston Consulting today to discuss how we can support your efforts in implementing ICH Q14 and elevate your analytical procedure development practices. Let us help you navigate the evolving regulatory landscape and ensure consistent quality in your drug manufacturing processes.