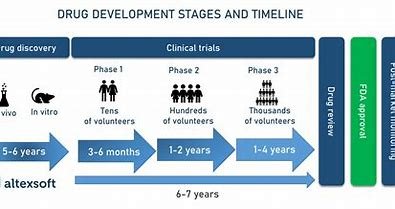
Discover strategies for accelerating drug development timelines, including early planning, clinical trial optimization, and expedited regulatory pathways. BioBoston Consulting can help streamline your process.
In the fast-paced world of drug development, accelerating timelines is critical to getting innovative treatments to patients faster. Whether you are developing small molecules, biologics, or gene therapies, reducing the time it takes to bring a drug to market can not only increase your competitive edge but also improve patient outcomes. At BioBoston Consulting, we specialize in helping biotech companies and pharmaceutical firms develop strategies to streamline drug development processes and achieve faster approval.
In this article, we will explore key strategies to accelerate drug development timelines, from early-stage research through to clinical trials and regulatory approval. We will also highlight how BioBoston Consulting can support your efforts with expert advice and actionable strategies.
1. Early Planning: Setting the Stage for Fast-Track Development
One of the best ways to accelerate drug development is to start with a clear plan that includes regulatory strategy, trial design, and resource allocation. Proper early-stage planning ensures that the development process moves forward smoothly and efficiently.
Key Considerations for Early-Stage Planning:
- Regulatory Engagement: Early discussions with regulatory bodies such as the FDA and EMA can help you define clear development pathways, identify potential risks, and gain advice on Fast Track or Breakthrough Therapy designations.
- Adaptive Trial Designs: Instead of traditional fixed clinical trial designs, consider using adaptive trial designs that allow for modifications based on interim results. This approach can help speed up the decision-making process during clinical trials.
- Preclinical Data Quality: Ensure high-quality preclinical data to minimize the chances of setbacks during clinical development. Solid preclinical results can pave the way for faster IND approvals and smoother trial execution.
By focusing on strategic planning early, you can address potential roadblocks before they arise, saving valuable time in the development process.
2. Leveraging Technology: Streamlining Drug Discovery and Research
Technology has revolutionized the drug discovery process, making it possible to analyze vast amounts of data, identify potential drug candidates faster, and optimize clinical trial designs.
Technologies for Accelerating Drug Development:
- Artificial Intelligence (AI): AI and machine learning models are now being used to identify drug candidates and predict clinical outcomes more efficiently than ever before.
- High-Throughput Screening (HTS): HTS allows researchers to rapidly test large libraries of compounds against specific biological targets. This speeds up the identification of potential drug candidates and reduces the need for lengthy, manual testing.
- Biomarker Identification: The identification and validation of biomarkers can help identify patients most likely to benefit from a drug, enabling more targeted and efficient clinical trials.
By leveraging cutting-edge technologies, you can accelerate the discovery phase and move your drug development process forward at an accelerated pace.
3. Optimizing Clinical Trials: Reducing Time Without Sacrificing Quality
Clinical trials often represent the longest phase of drug development, but there are several ways to optimize this process and reduce trial timelines while maintaining high-quality data.
Clinical Trial Optimization Strategies:
- Global Multi-Center Trials: Conducting trials in multiple regions can speed up enrollment and increase patient diversity, reducing overall study timelines.
- Real-World Evidence (RWE): Integrating real-world data into your trials can reduce the need for traditional trials by providing additional evidence of a drug’s safety and efficacy.
- Risk-Based Monitoring: Implementing a risk-based monitoring approach allows you to focus on high-risk areas during the trial and minimize unnecessary monitoring, which can save time and reduce costs.
- Patient Recruitment: Use data-driven strategies and digital tools to identify and recruit eligible patients more quickly. Patient recruitment delays are a major bottleneck in many trials, so improving this process can significantly speed up the overall timeline.
Optimizing clinical trial design, patient recruitment, and monitoring processes can significantly shorten the time it takes to complete this critical phase of drug development.
4. Regulatory Pathways: Fast-Tracking Drug Approval
In addition to early planning, the regulatory pathway you choose can have a significant impact on the speed of your drug’s approval. By working closely with regulatory agencies and utilizing expedited pathways, you can significantly accelerate your development timeline.
Expedited Regulatory Pathways:
- Fast Track Designation: The FDA’s Fast Track program is designed to speed up the development and review of drugs that address unmet medical needs. If your drug qualifies, it may benefit from frequent interactions with the FDA and priority review.
- Breakthrough Therapy Designation: For drugs that show substantial improvement over existing therapies for serious or life-threatening conditions, the Breakthrough Therapy designation provides a streamlined development and review process.
- Rolling Review: A rolling review allows you to submit sections of your New Drug Application (NDA) or Biologics License Application (BLA) as they are completed, rather than waiting until all data is available, which can shorten the approval process.
By exploring and utilizing these expedited pathways, you can speed up the approval process and get your product to market faster.
5. Post-Market Strategy: Accelerating Market Access and Adoption
Once your drug is approved, the work is not over. Ensuring that your drug reaches patients quickly after approval is key to maximizing its impact. A strong post-market strategy can help accelerate patient access and adoption.
Post-Market Strategies for Accelerated Drug Access:
- Market Access Strategy: Develop a comprehensive market access strategy that includes reimbursement negotiations, pricing strategies, and health economics data to ensure quick adoption by healthcare providers and payers.
- Patient Support Programs: Implementing patient assistance programs can improve access for patients and increase the drug’s visibility in the market.
- Ongoing Monitoring: Post-market surveillance ensures that your drug continues to meet safety standards and that adverse events are quickly addressed.
By having a clear post-market strategy, you ensure that your drug is not only approved but also widely accessible to patients as quickly as possible.
6. How BioBoston Consulting Can Help Accelerate Your Drug Development Timelines
At BioBoston Consulting, we specialize in helping biotech companies and pharmaceutical firms accelerate their drug development timelines. Our team of experts can help you navigate every stage of the process, from early-stage planning to regulatory submissions, clinical trials, and post-market strategies.
How BioBoston Consulting Can Assist:
- Regulatory Strategy: We can help you identify and pursue expedited regulatory pathways, ensuring your drug receives the attention it needs for faster approval.
- Clinical Trial Design: Our experts can help optimize your clinical trial designs, from adaptive trial strategies to global multi-center trials, to reduce time and costs.
- Technology Integration: We provide advice on integrating the latest technologies into your development processes, ensuring you remain at the forefront of innovation.
- Market Access and Post-Market Strategies: We help you design comprehensive market access and post-market strategies to ensure your drug reaches patients quickly and efficiently.
By partnering with BioBoston Consulting, you gain access to the expertise and resources needed to accelerate your drug development timelines and bring your innovative treatments to market faster.
7. Conclusion: Accelerate Your Drug Development with BioBoston Consulting
In the competitive world of drug development, time is of the essence. By implementing strategies to optimize every stage of the process—from early planning to regulatory approval and post-market access—you can reduce timelines and ensure that your product reaches patients faster. BioBoston Consulting is here to guide you through each stage of development, helping you accelerate your journey from discovery to market.
Ready to accelerate your drug development timelines? Contact BioBoston Consulting today to discuss how we can help streamline your development process and get your drug to market faster.