“Discover how GMP training fosters regulatory compliance, enhances product safety, and cultivates a quality culture in the life science industry.”
In the process, you will find out how Good Manufacturing Practices (GMP) training principles carved the backbone to maintain industry standards and establish a culture of best practices yet compliance.
Introduction: The Importance of GMP in Life Sciences
In the life science realm, we are at the forefront of creating breakthrough products that saves lives and improve quality of life.
The World of International Standards without borders is a fragile vessel, and the pursuit of scientific progress is not exempt from caring if the consumer goods are safe and good for health. That is where the Good Manufacturing Practices (GMP) principles are included.
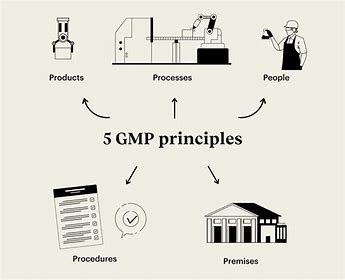
Overview of Good Manufacturing Practices (GMP)
GMP, which is a regulation of the FDA, EMA or PMDA, and other regulating agency maintain that to keep product integrity at the highest standards possible in life science industry.
This outlines a detailed best practice procedure from raw material control to product labeling.
Why GMP Training is Essential
Realizing the crucial importance of following GMP guidelines, it is clear that all employees who are part of production process need to have a comprehensive knowledge of these directives.
The cornerstone of GMP training is making sure employees understand compliance with the numerous regulations. The consequences of non-compliance can be costly, from product recalls and financial penalties to criminal charges.
Compliance Training Focused on your Organization
It is of a critical importance to tailor the GMP training offering to satisfy employees´ wide ranging needs. Everyone from the operators to the quality control are playing an important part in ensuring GMP is of paramount focus. Training will cover a wide range of essentials, from hygiene guidelines, proper documentation methods, and equipment to quality controls.
The training program needs to be updated on a regular basis due to the fast regulatory and industry best practices changes.
More than Compliance: A Far-reaching Effect
Understand GMP training mainly concerns with regulatory compliance, but the influence of it stretches much more than just implementing to guidelines. It is an important mechanism to create a culture of safety and quality in the organization. It can serve to energize employees and increase the intrinsic value of their work if it simply reminds them that this represents a set of core values we want to continue fostering regardless of how tough or challenging our competition currently is.
Additionally, by having an in-depth understanding of GMP practices, employees can identify issues beforehand during the manufacturing process and mitigate them, which in turn improves overall productivity.
Why? The Necessity of Thorough GMP Training
In the rapidly changing life science industry land scape, this very important check on product integrity is fundamental. Putting effort into investing in high quality GMP training programs is a statement from company ownership which really shows safety and quality cannot be compromised.
Given the fast pace of change in regulations and industry best practices, you want to make sure your employees stay up to date so they have the knowledge and skills needed to do their jobs safely and effectively. “
Conclusion: Commitment to Safety and Quality
To sum it up, GMP training is a modus operandi for ensuring safety and quality with the respective regulatory compliances within a very volatile Life Science Industry. It is not just a token set of conduct but an ethic ensuring continuous improvement and consumer safety.
Find out how BioBoston Consulting can help you: contact us or check out our website.