Explore the latest advancements in immunotherapy, focusing on CAR T cell therapies. Discover the importance of analytical testing, flow cytometry, and the regulatory landscape in ensuring the safety and efficacy of these innovative cancer treatments.
CAR T cell therapies have captured much attention and hope on the part of researchers, clinicians and patients. The results from the initial clinical trials were striking and have since led to approval for B-cell leukemia and lymphoma indications. These treatments have demonstrated high response rates and at times durable remission offering an option for those patients where standard available therapies failed.
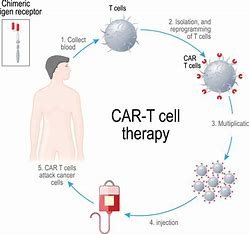
Over the past few years, the field of immunotherapy has made incredible progress and has literally changed the way we think about treating cancer. One of the most exciting interventions in this space are Chimeric Antigen Receptor T-cell (CAR T cell) therapies. These cutting-edge therapies work by using a patient’s own immune system to fight cancer, essentially programming their T cells with CARs. CAR-mediated recognition allows T cells to selectively identify and kill cancer cells and thus this presents a targeted, cytotoxic approach.
The early success of CAR T cell therapies is highly encouraging, but the development and clinical application of these therapies are challenging since they require robust analytical testing. Analytical testing is a critical aspect of the development process for new therapeutic products, as data generated from these studies directly impact conclusions regarding the levels of safety, quality and efficacy. Regulatory bodies like the U.S. Food and Drug Administration (FDA) now require thorough analytical testing to demonstrate that key attributes such as product identity, purity, potency, and safety meet some defined standards.
Early Assay Development and Validation is the New Order
Because of the intricacy of CAR T cells and the numerous methods by which they have a healing result, certain biological assays precisely give info on the analyzed item. Like any therapy product, initial assay development is an essential step to successfully advance to early preclinical and clinical testing. Pushing the development of an assay to later in the development process can ultimately cause delays and hamper evaluation of these therapies, thus potentially preventing life-saving treatments getting to patients in a timely manner.
The validation of the assay is an important part for analytical testing. Validations are needed in order to prove that results from a test are accurate and reliable in the pharmaceutical sector. In Phase 1 studies, formal validation is not necessary but is the guideline for test methods that must be controlled by data. In doing this, researchers and sponsors can inspire greater confidence in the scientific community and regulatory agencies that clinical studies are ready to move forward.
Analytical assays should follow valid scientific principles – including specificity, sensitivity (and limits of recovery), precision and reproducibility. This involves choosing valid controls and standards to get results within an acceptable level of accuracy and precision. When available and particular in the context, compendial methods should be pursued, with safety-related tests qualified prior to starting clinical studies.
With the advancement of CAR T cell therapies down the development pipeline, each assay should be qualified to suit the needs of various stages. For example, if an assay is intended as the only way to demonstrate primary evidence of effectiveness in support of a marketing application, the test must be well-characterized. Analytical test results are needed for BLA filings to show that the therapy is both safe and effective and can be approved for market.
Flow Cytometry: A Useful Tool in CAR T Cell Testing
Flow cytometry is one of the many analysis techniques used to evaluate engineered CAR T cells as it has shown its power in detecting and measuring wild type T cell properties. The high parameter flow cytometry provides valuable information about different characteristics of CAR T cell at every stage of the manufacturing process. These are the critical assay parameters that can be measured using flow cytometry for assessing cell viability, identity, purity, and potency necessary for safe and effective CAR T cell therapies.
Reproducibility and reliability of these assays show that they are developed logically, by following scientifically sound principles. Validated flow cytometry assays, when done correctly, provides one of the most accurate and consistent ways to evaluate CAR T cell characteristics which facilitates decision-making throughout preclinical and clinical development.
The INDA provides complete details of the flow cytometry assay prior to the first submission. This description should provide the antibody panel and gating strategy utilized to define cell populations, as well as confirmation that live/dead staining for each relevant cell population was included in the final product. Careful instrument calibration and quality control programs are essential to ensure accurate flow cytometry results.Assay controls are essential in flow cytometry to verify the accuracy of data.
Compensation controls: they are means of using the compensation values in order to adjust for spectral overlap between fluorochromes, so that results obtained can be correctly interpreted. Fluorescence Minus One (FMO) controls establish the level of fluorescence spread and gates for marker-negative populations, and isotype controls serve to define nonspecific binding.
Appropriate sample staining, acquisition, and data analysis are also important for assay development to get high-quality outputs. These should also lead to consistency and standardization within the different laboratories and users.
For CAR T cell analytical testing, the direct detection of the CAR is important since this is an essential part of flow cytometry. The percentage of CAR+ cells is important to know the extent of production efficiency in CAR T cell. If a surrogate marker is used to determine CAR expression, demonstrating with the sensitivity and specificity of the surrogate marker will be required for validation as an alternative detection method.
As outlined in the guidelines of the International Conference on Harmonization (ICH) Q2, a full validation study should be performed for flow cytometry used in lot release. This verification study includes verifying each of the fluorescently labelled markers in the flow cytometry panel on the flow cytometer(s) used for CAR T cell release. Such validation also includes evaluation of the maximum time samples can be held before staining or after certain steps in order to better guide robustness studies. In addition, records of training for all users who conducted the validation studies should be recorded to secure consistency and reproducibility of such assay.
Deconstructing Vector Copy Number (VCN) to Optimize Safety
Vector Copy Number (VCN): A critical component of CAR T cell analytical testing includes the calculation of VCN, which is the number of copies average insertion transgene per total number of receptors lash by a single cell. CAR T-cell transgene integration can impact endogenous cellular gene expression leading to tumorigenicity in certain cases. Accordingly, VCN determination is a critical need for screening the safety of CAR T cell therapies.
Therefore, VCN calculated as a function of CAR-expressing cells gives a more accurate reflection of the VCN in transduced cells as it provides an indication of safety by estimating the possibility for insertional mutagenesis. The release criterion for the VCN is key to establishing product specifications and maintaining product quality throughout clinical development of CAR T cell therapies.
Legal and Security Compliance: The VCN release criterion is heuristic stemming from experience backed by a detailed risk assessment. The risk assessment might include a revision of previous studies to understand insertional-site analysis, clonal dominance, dose-response relationships, enlightenment, or depths of effects in certain disease models tested etc. Experimental data from multiple engineering manufacturing runs can provide supporting evidence for the risk assessment used in developing VCN release specification
It may be difficult for expediters of CAR T cells produced with short culture duration to measure VCN stably integrated at the time of lot release testing when episomal copies of non-integrated vectors are present. Here in these cases, it may be required to have interim VCN assessments at the time of lot release followed by subsequent VCN assessments on cultured CAR T cells to avoid erroneously determining and stably integrated VCN.
Identity Testing: Be Specific and Consistent
Analytical testing for CAR T cells includes identity (which is comprehensive and performed throughout), which must be performed for every stage of development space and licensure agents. Identity testing is to confirm unequivocally that a CAR T cell product can be accurately identified and differentiated from other products produced at the same site. Tests need to be specific and consistent to demonstrate with confidence that CAR T cell therapies are identical.
Identity testing for CAR T cells should be performed using assays that detect the presence of the transgene (e.g., flow cytometry or Polymerase Chain Reaction [PCR] to measure CAR expression. In addition, cellular composition of the final products including cell surface markers should be evaluated by specific assays. The assay is designed to be orthogonal to the other assays. If all three show tests show positive signals for CAR, then there is more confidence that the final product is indeed a CAR T cell product.
It is important to note that Human Leukocyte Antigen (HLA) typing may be done for autologous CAR T cells. Yet, as the genetic modulation that would CAR expression introduces is not detected by HLA typing, this is an insufficient identity test. Therefore, the need for chain of identity to verify product traceability in CAR T cell therapies cannot be eliminated by HLA typing.
Efficacy Testing: Pathway Investigation
The potency assay is a critical factor in determining the clinical effectiveness of CAR T cell therapies. The therapeutic effects of CAR T cells are mediated primarily through killing of target cells, secretion of cytokines and activation of immune cell. Hence, proper assessment of potency is critical for accurately evaluating the clinical efficacy of CAR T cell therapies.
A matrix power measurement approach is suggested, other ways evaluated CAR T cell strength are in orthogonal. These experiments may extend from cell killing assays to analyze the ability of CAR T cells to kill target cells, transduction efficiency experiments measuring the proportion of successfully genetically modified cells and even functional cytokine secretion analysis to investigate potential immunomodulatory effects exhibited by the engineered CAR T cells.
Each functional element should have a separate potency assay per transgene component for CAR T cells that express more than one transgene component. For example, if a CAR T cell also entails the inclusion of other transgenes unrelated to the CAR itself (i.e., “secondary” or passenger genes) like cytokine transgenes – then separate potency assays should be established for each functional component.
CAR T cell therapies are so exciting because they offer the potential to completely change the previously grim outlook for many patients with otherwise refractory and life-threatening diseases. To tap into their full potential, these therapies must undergo thorough analytical testing to make sure they meet safety, efficacy and quality standards.
The CAR T cell process Early assay development and validation are critical for generating robust data during each phase of the CAR T cell development process. Immune cell population assessments by flow cytometry allow different facets of CAR T cell function to be studied separately and thus help make decisions about what parameters need further development during preclinical or clinical development.
Vector Copy Number (VCN) is a critical parameter for the quality control of CAR T cell therapeutics and to establish product specifications. The rigorous testing of identity is important to confirm the specificity and constancy of CAR T cell products, while detailed potency evaluation allows for delineation of mechanisms generating therapeutic ability of these innovative therapies.
Conclusion
Moving forward, the developments in analytical testing methodologies inherently triggers more effective and safer CAR T cell therapy. Through rigorous analytical testing, researchers, clinicians and manufacturers are collectively pushing the CAR T cell therapy field forward and giving patients everywhere hope for brighter and better future.
To find out more about what we can do for your organization, please contact BioBoston Consulting today or visit our website.